China’s Leading Role: Pharmaceutical Impurities, Standards, and APIs
In the pharmaceutical industry, the quality and purity of medications are of paramount importance. China stands as a hub for pharmaceutical impurities suppliers, impurity standard manufacturers, and active pharmaceutical ingredient (API) production, playing a pivotal role in global healthcare standards.
Pharmaceutical Impurities Suppliers in China:
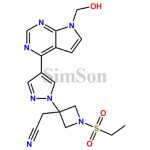
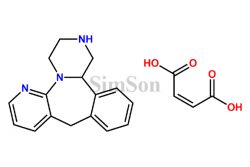
Pharmaceutical impurities, albeit unwanted, are an inherent part of drug development and manufacturing. Specialized suppliers in China provide pharmaceutical impurities for research, development, and quality control purposes. These suppliers offer a range of impurities for various stages of drug development, aiding pharmaceutical companies in identifying, analyzing, and developing solutions to ensure the purity and safety of their medications.
Impurity Standard Manufacturers in China:
Impurity standards serve as crucial reference points for pharmaceutical companies. Manufacturers in China specialize in producing these standards, allowing for the accurate identification and quantification of impurities in pharmaceutical products. These standards aid in maintaining the quality, safety, and efficacy of medications, ensuring compliance with stringent regulatory requirements.
Active Pharmaceutical Ingredients (APIs) in China:
China is a key player in the production of active pharmaceutical ingredients, the core components responsible for the therapeutic effects of medications. Chinese manufacturers are known for their expertise in producing high-quality APIs, adhering to international quality standards. The country’s robust infrastructure and skilled workforce enable the efficient and cost-effective production of APIs, contributing significantly to the global pharmaceutical market.
Conclusion:
The pharmaceutical industry in China thrives on a network of pharmaceutical impurities suppliers, impurity standard manufacturers, and active pharmaceutical ingredient producers. These entities play a crucial role in maintaining pharmaceutical quality, safety, and regulatory compliance. Their contributions are instrumental in the development and manufacturing of medications that meet stringent global healthcare standards.