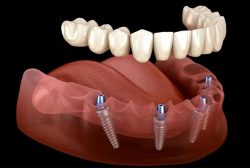
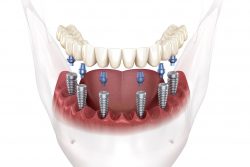
510k submission
FDA 510(k) Clearance – The FDA 510(k) clearance is a premarket submission made to the FDA to demonstrate that the device to be marketed is at least as safe and effective, that is, substantially equivalent, to a legally marketed device (predicate device). This process is required for most medical devices in the United States before they can be legally marketed. The submission contains data comparing the device to the predicate device, demonstrating its safety and effectiveness. Once cleared, the device can be marketed in the United States.
510(k) Submission – A 510(k) submission is a premarket submission made to the FDA to demonstrate the device’s substantial equivalence to a predicate device. It typically includes technical, scientific, and clinical data and information about the device. The submission aims to demonstrate that the device is as safe and effective as the predicate device and meets the FDA’s regulatory requirements.
CE Marking for Medical Devices – CE marking is a certification mark that indicates conformity with health, safety, and environmental protection standards for products sold within the European Economic Area (EEA). For medical devices, CE marking indicates compliance with the European Union’s Medical Device Regulation (MDR) or the In Vitro Diagnostic Regulation (IVDR), depending on the type of device. CE marking allows manufacturers to legally market their medical devices in the European market.
If you have any specific questions about these topics or need further information, feel free to ask!
Visit for more information – https://www.i3cglobal.com/medical-device-ce-marking/