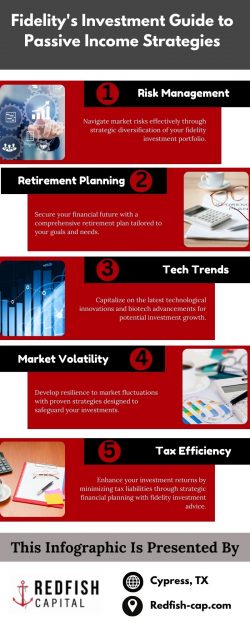
Clinical Study Report Translation; CSR Translation
Clinical Study Reports (CSRs) are critical means by which regulators can assess the outcome of clinical studies. Their format and content are defined in Guideline for Industry Structure and Content of Clinical Study Reports (ICH E3). A clinical study report must reflect the detail and scientific rigor accurately. CSRs also include Clinical Trial Reports – the new EU terminology for CSRs when the study report pertains to an interventional (rather than non-interventional) clinical trial. The trial subject documentation has unique translation requirements. MedTrans has experience translating content for clinical study reports destined for a variety of regulatory markets. From recruiting documents to surveys and evaluations, documents that address patients and subjects directly must and will be translated professionally by our certificated translators.