ECHEMI | Magnesium sulfite
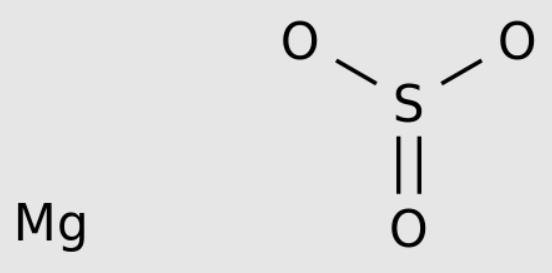
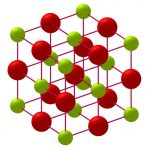
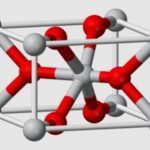
Magnesium sulfite is the magnesium salt of sulfurous acid with the formula MgSO 3. Its most common hydrated form has 6 water molecules making it a hexahydrate, MgSO 3·6H 2O. When heated above 40°C (104°F), it is dehydrated to magnesium sulfite trihydrate, or MgSO 3·3H 2O. The anhydrous form is hygroscopic, meaning that it readily absorbs water from the air.