Stability Study
Stability Study
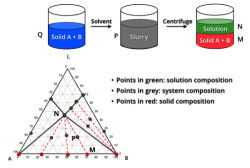
Stabilities studies, regardless of preformulation, early development, or late stages, are critical for formulation development. We will provide stability services for each development stages, including protocol design and testing of accelerated stability studies at preform and prototype development, probe and IND stability for RED, stability study in late stage, e.g. formal stability study (FSS), product characterization study (PCS) for packaging definition, stability of intermediates (e.g. spray dried intermediates, SDI), the hold-time stability of the drug products in production, in-use stability testing, stability of storage conditions and photostability testing based on ICH Q1A/Q1B, reevaluation of stability beyond the expiry date, and stability study of drug products after commercial launch etc. These stability data will provide strong evidence for packaging selection, expiration date setting, and lifecycle stability evaluation of drug products.