API Form and Solid-State Research
Highlights of API Form and Solid-State Services
Serving over 800 clients worldwide
Supported solid form screening, crystallization, and preclinical formulation development of over 1,500 new drug molecules
The world’s leading technology supports the understanding of molecule and formulation with a high-end platform
High-quality service, a culture of confidentiality, and fast turnaround with cost-effective pricing
Customized solution for your molecule, spanning the entire drug lifecycle
Highlights of API Form and Solid-State Services
API Form and Solid-State Services
Solid Form Screening and Selection
Vetting of API solid forms for clinical studies is critical to maintain the integrity of downstream processes. Crystal Pharmatech makes best-in-class decisions utilizing a data-driven, multi-disciplinary approach based on institutional knowledge and comparative analysis.
Amorphous Solid Dispersions
Low water-soluble compounds can exhibit bio-performance issues such as poor PK exposure or non-linear PK exposure. To improve the bio-performance, amorphous solid dispersions of amorphous drug stabilized in polymer excipients are commonly developed for solubility enhancement.
Absolute Structure Determination
The physicochemical properties of a compound is closely related to its structure and often can be predicted from its structure. Single crystal X-ray data collection and structure elucidation is one of the best techniques to obtain the 3-dimentional structural information, including bond connectivity, chirality, solvate/water participation etc.
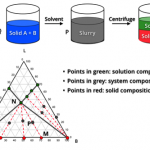
Crystallization Screening and Development
Crystallization is a critical unit operation in API manufacturing for both final product and intermediates. Desired product quality attributes can be obtained economically with high efficiency through well-designed and controlled crystallization processes.
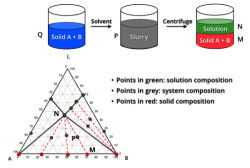
Chiral Separation by Crystallization
More than 70% of drug candidates worldwide are chiral. Typically, for chiral API, only one enantiomer or diasteromer is biologically active or desirable. Therefore, the production of enantiopure compounds or diastereomers is imperative. In the production of small molecule drugs with desired chirality, separation via crystallization can be much more economical and environmentally favorable than chromatography.
Solid-State Characterization (FAST)
Our Solid State Characterization Service, which is referred to as Focused Analytical Solids Testing (FAST), covers many routine analyses encountered during pharmaceutical development. The FAST concept is directly aligned with our goal of partnering with organizations instead of merely providing data.
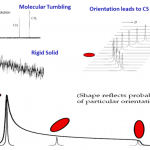
Solid-State NMR Analysis
Nuclear Magnetic Resonance (NMR) Spectroscopy is a ubiquitous analytical technique utilized across a wide range of disciplines.