Formulations for PK/Efficacy/Tox Studies
Formulations for PK/Efficacy/Tox Studies
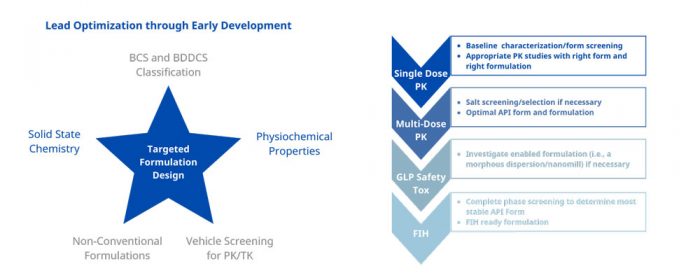
Contrary to popular belief, early formulation development is not simply dumping API in a vehicle and gavage feeding an animal. If you do this, you will most likely lose time and money with unnecessary and confusing PK studies. This led to the development of our SMART biopharmaceutical solutions that offer input from our scientific advisory board: A one-stop shop from candidate selection through GMP FIH.
This is an integrated approach to finding the right form and formulation at every step of the development cycle using decision trees and guidance documents built from our over 80 years of innovative pharmaceutical experience. Using our SMART services will lead to the shortest possible development timelines with limited resource utilization.
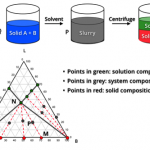
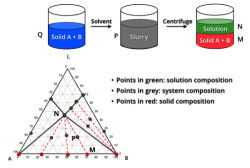
Our formulation approaches to deliver molecules to support PK/Efficacy/Tox Studies include solution and suspension formulation selection through vehicle screening, solubilization through pH adjustment, co-solvents, surfactants, cyclodextrin, in-situ salt formation. In addition, we also apply special drug delivery technologies for insoluble compounds.
■ Solution/Suspension Formulations
■ Solubilization through pH Adjustment, Co-solvents, Surfactants, Cyclodextrin, In-situ Salt Formation
■ Special Drug Delivery Technologies
● Amorphous Solid Dispersion
● Particle Size Reduction
● Nano-milling: Nano-suspension/powder
● Micronization by Jet-milling
● Lipid-based Formulation
● Self-emulsifying Drug Delivery Systems (SEDDS)
● Liposome